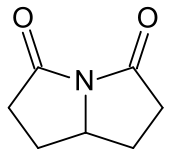
Rolziracetam
Appearance
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 2,6,7,8-tetrahydro-1H-pyrrolizine-3,5-dione, CI 911 & Lukes-Šorm dilactam. |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C7H9N2O2 |
| Molar mass | 153.161 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Rolziracetam is a nootropic drug of the racetam family.
Rolziracetam was found to improve performance on a delayed-response task in aged rhesus monkeys. It has a wide margin of safety in animals and has been evaluated for use in cognitively impaired human subjects.[1]
Synthesis[edit]

- dimethyl 4-nitropimelate [7766-83-8] (1)
- methyl 3-(5-oxopyrrolidin-2-yl)propanoate [81980-11-2] (2)
- 3-(5-oxopyrrolidin-2-yl)propanoic acid [7766-86-1] (3)
See also[edit]
- Piracetam
- Rolziracetam is used as precursor to make a compound called CI-933 [91829-95-7] (another racetam).
References[edit]
- ^ a b Butler DE, Leonard JD, Caprathe BW, L'Italien YJ, Pavia MR, Hershenson FM, Poschel PH, Marriott JG (March 1987). "Amnesia-reversal activity of a series of cyclic imides". Journal of Medicinal Chemistry. 30 (3): 498–503. doi:10.1021/jm00386a010. PMID 3820221.
- ^ Lukeš, R.; Šorm, F. (1947). "Sur les acides aminodicarboxyliques symétriques et leur cyclisation". Collection of Czechoslovak Chemical Communications. 12: 278–291. doi:10.1135/cccc19470278.
- ^ Micheel, Fritz; Flitsch, Wilhelm (1955). "Eine einfache Synthese des 3.4-Dioxo-pyrrolizidins (Pyrrolizidinderivate III.)". Chemische Berichte. 88 (4): 509–510. doi:10.1002/cber.19550880410.
- ^ Micheel, Fritz; Flitsch, Wilhelm (1956). "Synthesen mit 3.4-Dioxopyrrolizidin (IV. Mitteil.)". Chemische Berichte. 89 (1): 129–132. doi:10.1002/cber.19560890121.
- ^ Alonso, Ricardo; Gessner, Wieslaw; Takahashi, Kimio; Brossi, Arnold (1988). "Improved Synthesis of the Lukes-ŜOrm Dilactam. Nucleophilic Opening to 5-Substituted 2-Pyrrolidine-2-Ones". Synthetic Communications. 18 (1): 37–43. doi:10.1080/00397918808057817.
- ^ Donald E. Butler, U.S. patent 4,372,966 (1983 to Warner Lambert Co LLC).
- ^ Herman A Bruson, U.S. patent 2,390,918 (1945 to Resinous Prod & Chemical Co).
- ^ Marvin S. Hoekstra, U.S. patent 4,663,464 (1987 to Warner Lambert Co LLC).
